caged-Ins(145)P3 / PM
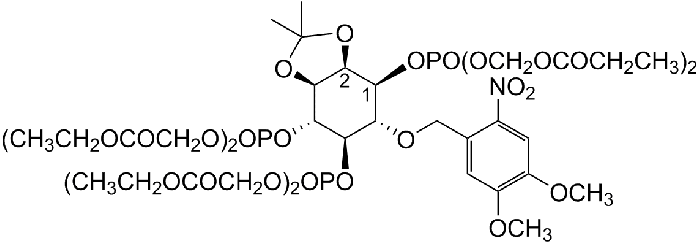
D-23-O-Isopropylidene-6-O-(2-nitro-4.5-dimethoxy)benzyl-myo-Inositol 145-trisphosphate-Hexakis(propionoxymethyl) Ester
photoactivatable AND membrane-permenat IP3 derivative
In stock
Catalogue
cag-iso-2-145-100
Size: 100 µg
€650.00
| Catalogue | cag-iso-2-145-100 |
|---|---|
| Formula | C42H64NO31P3 |
| CAS | 1009832-82-9 |
| MW | 1171.87 |
| Appearance | oil |
| Purity | > 98% |
| Solubility | in DMSO. CH2Cl2. Me |
| Size | 100 µg |
| Storage | Keep cool and dry. Protect from light and moisture. |
<p><strong>Membrane-permeant derivatives of inositol phosphates </strong>require the intracellular enzymatic hydrolysis of several protecting groups in order to generate the biologically active compound. The photolysis of membrane-permeant caged derivatives of Ins(145)P3 might mimic fast intracellular responses. In an initial step <strong>cells are loaded with the caged Ins(145)P3 derivative</strong>.</p>
<p>Within 30 to 180 minutes all bioactivatable protecting groups remove generating <strong>caged inositol phosphate</strong>. The cage is known to prevent biological activity. The photochemical destruction of the cage (360-400nm) releases active Ins(145)P3 within a few seconds. Since this approach does not (directly) trigger other signaling events, membrane-permeant derivatives of signaling molecules are able to help dissecting <strong>signaling pathways</strong>.</p>
We found other products you might like!
-
 caged-Ins(145)P3 / PM€750.00
caged-Ins(145)P3 / PM€750.00 -
 caged-Ins(145)P3O6€650.00
caged-Ins(145)P3O6€650.00 -
 caged-Ins(145)P3 P4€650.00
caged-Ins(145)P3 P4€650.00