caged-Ins(145)P3 / PM
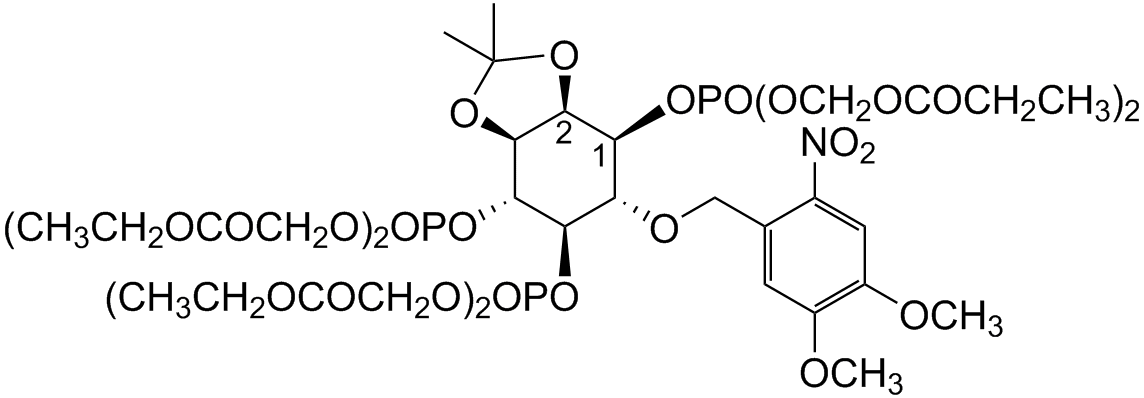
D-23-O-Isopropylidene-6-O-(2-nitro-4.5-dimethoxy)benzyl-myo-Inositol 145-trisphosphate-Hexakis(propionoxymethyl) Ester
photoactivatable AND membrane-permenat IP3 derivative
| Catalogue | cag-iso-2-145-10 |
|---|---|
| Formula | C42H64NO31P3 |
| CAS | 1009832-82-9 |
| MW | 1171.87 |
| Appearance | oil |
| Purity | > 98% |
| Solubility | in DMSO. CH2Cl2. Me |
| Size | 10 * 10 µg |
| Storage | Keep cool and dry. Protect from light and moisture. |
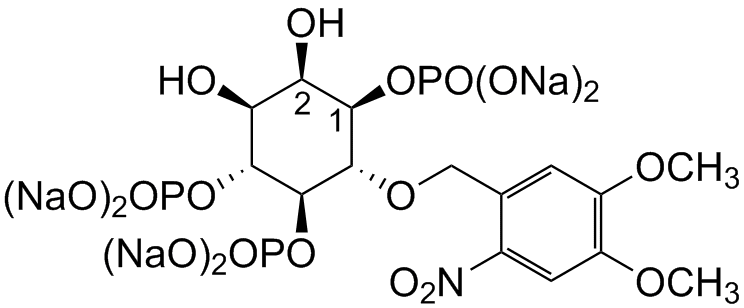
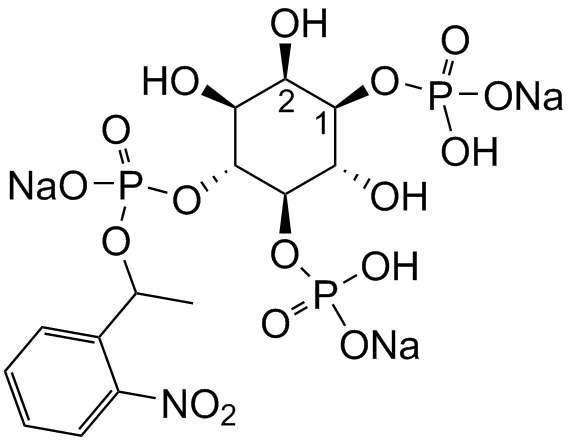
Membrane-permeant derivatives of inositol phosphates require the intracellular enzymatic hydrolysis of several protecting groups in order to generate the biologically active compound. The photolysis of membrane-permeant caged derivatives of Ins(145)P3 might mimic fast intracellular responses. In an initial step cells are loaded with the caged Ins(145)P3 derivative.
Within 30 to 180 minutes all bioactivatable protecting groups remove generating caged inositol phosphate. The cage is known to prevent biological activity. The photochemical destruction of the cage (360-400nm) releases active Ins(145)P3 within a few seconds. Since this approach does not (directly) trigger other signaling events, membrane-permeant derivatives of signaling molecules are able to help dissecting signaling pathways.